Basic HTML Version


I primi modelli atomici
UNITÀ
C1
13
7.
La composizione isotopica dell’ossigeno è:
8
16
O (99,759%),
8
17
O (0,037%),
8
18
O (0,204%), con rispettive masse atomiche
15,9949, 16,9991 e 17,9992. Calcola la massa atomica media
dell’ossigeno.
8.
Il carbonio naturale ha massa atomica 12,011 ed una mi-
scela di due isotopi di massa 12,000 (
6
12
C) e 13,0034 (
6
13
C ).
Quali sono le percentuali dei due isotopi?
9.
La massa atomica del boro è 10,812, sapendo che è costituito
da due isotopi di massa 10,013 e 11,009; calcola l’abbondan-
za percentuale di ciascuno di essi.
La miscela isotopica
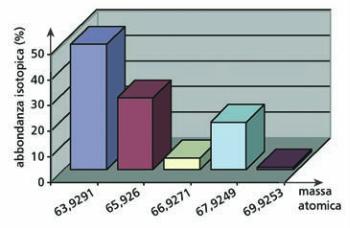
Lo zinco è una miscela di cinque isotopi, presenti in natura ciascuno in percentuali diverse.
La percentuale di ciascun isotopo (
abbondanza isotopica
) è nota (Tabella 3): si vuole
ricavare la massa atomica dello zinco (massa
Zn
).
Soluzione
massa
Zn
=
⋅
+
⋅
+
63 9291 48 89 65 9260 27 81 66 9271
,
,
,
,
,
⋅
+
⋅
+
⋅
4 11 67 9249 18 57 69 9253 0 62
100
,
,
,
,
,
massa
Zn
=
65,3869
Una miscela semplice
Il cloro ha massa atomica 35,453 ed è una miscela di due isotopi,
17
35
Cl e
17
37
Cl , le cui masse
atomiche sono rispettivamente 34,969 e 36,966. Calcola l’abbondanza percentuale dei due isotopi.
Soluzione
Indicando con
x
e 100
-
x
le percentuali dei due isotopi si ha: 35 453
34 969 36 966 100
100
,
,
,
=
⋅ +
⋅
−
(
)
x
x
Da cui
x
=
75,76; quindi:
%
17
35
Cl
=
75,76
e
%
17
37
Cl
=
24,24
esempi
!
Ricorda che facciamo riferimen-
to alla
massa atomica relati-
va
(Unità 5).
Chemistry Readings
The Mass Spectrometer
Mass spectrometry is an analytical technique to identify the chemical
composition of a compound or sample based on the mass-to-
charge ratio of charged particles. A sample undergoes chemical
fragmentation forming charged particles
(ions). The mass-to-charge ratio of the
particles is calculated by passing them
through electric and magnetic fields in a
mass spectrometer.
How does it work?
A mass spectrometer is made up of three
modules:
an
ion source
, which transforms
the molecules in a sample into ionized
fragments; a
mass analyzer
, which sorts the ions by their masses
by applying electric and magnetic fields; a
detector
, which
measures the quantities of each ion fragment and then provides
for calculating the formula of the compounds.
In the ion source, the sample is vaporized and ionized. The speed
of a charged particle may be increased or decreased while passing
through the electric field and its direction may be altered by the
magnetic field. The magnitude of the deflection of the moving
ion trajectory depends on its mass-to-charge ratio, lighter ions
get deflected by the magnetic force more than heavier ions.
The streams of sorted ions pass from the
analyzer to the detector, which records
the relative abundance of each ion type.
Why to use it?
This information is used to determine
the chemical element composition of the
original sample. The technique has both
qualitative and quantitative uses, such
as identifying unknown compounds,
determining the isotopic composition of elements in a compound,
the structure of a compound by observing its fragmentation,
quantifying the amount of a compound in a sample and determining
other physical, chemical, or biological properties of compounds.
Fill in the blanks
A mass spectrometer is composed of
…………....................……
. The speed of a charged participle can change according to
……….....................……
.
The mass spectrometer is used to determine the chemical
…………..............…………..
and physical, chemical, or biological
………….............................
of compounds. The sample is
………….............................
by applying electric and
………….............................
.
+
ionisation electric field
acceleration
deflection
magnetic
field
detector
to vacuum pump
vaporised
sample
Verso le competenze
XII1-029_U07_C1_Chimica_Tottola_CS4-sec imp.indd 13
12/12/11 1

